How to Determine Which Layer Is Aqueous Using Kitchen
To determine which layer is which one can simply add distilled water to the funnel. Water is more dense than ether and sinks to the bottom while the ether sits on top.

A The Kenwood Bl370 Series Kitchen Blender Used In This Work Download Scientific Diagram
How many 100 mL chloroform extractions would be required to extract at least 90 of Y from a 500 mL aqueous solution of water.
. Who are the experts. This is the best answer based on feedback and ratings. Water is more dense than ether and sinks to the bottom while the ether sits on top.
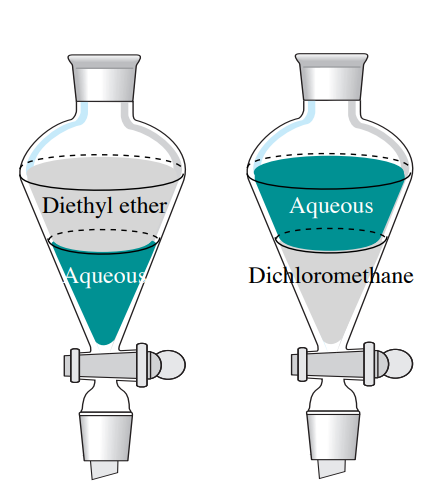
What two ways could you use to determine which layer is the organic layer and which is aqueous layer. In the right separating funnel the aqueous layer is on the top meaning the organic layer must be more dense than water. See the answer See the answer done loading.
Calculate the amount of 6 M HCl needed to neutralize the basic solution. At this point the two layers can be separated into their respective beakers. So the organic layer Compounds were trying to separate insoluble solvent.
Without tasting smelling or removing either layer from the container how can you determine which layer is the aqueous layer using just material found in a typical kitchen. Slowly add 6 M HCl until the pH of the aqueous layer is 2. Add a bit of water from a squirt bottle to the separatory funnel Figure 49a and watch where the water droplets go.
See the answer. In the left separating funnel the aqueous layer is on the bottom meaning the organic layer must be less dense than water. If the top layer is aqueous the water droplets should mix with the top layer and they will look as if they disappear.
You assume that this biphasic mixture has an aqueous layer and some type of organic layer. While stirring slowly add the 6 M HCl solution to the chilled solution. Place the basic aqueous solution in an ice bath.
100 1 rating by dis. See the answer See the answer done loading. See the answer.
6 Compound Y has a distribution coefficient of 40 when extracted from water with chloroform with Y being more soluble in chloroform. Whichever layer increases in size must be the aqueous layer and the other is the organic layer. If unsure which layer is aqueous and which layer is organic do one of the following things.
Look at the table on the previous slide. Suggest two methods for determining which layer in an extraction is the aqueous layer and which layer is the organic layer. To determine the aqueous layer-The NaHCO3 solution is an aqueous solution water is the solvent.
Use pH paper to determine the pH of the chilled aqueous solution. The organic layer also contains a solvent CH2Cl2 or ether that is insoluble in water. There is the organic layer which is insoluble in water and initially contains all of your compounds that you will eventually separate.
View the full answer. Previous question Next question. 4 points Calculations Answer 7 As you are cleaning out the shared refrigerator in the kitchen of your.
You Have A Mixture Of P Dichlorobenzene And Benzoic Acid Can You Draw A Flow Chart To Show How The Two Compounds Of This Mixture Could Be Separated Quora

Work Flow For First And Second Extractions A The Rna And Denaturing Download Scientific Diagram

Illustration Of Exfoliating Graphite In Aqueous Solutions Of Proteins Download Scientific Diagram
Comments
Post a Comment